Term Used to Describe Probabol Location of Electrons
Or rather the probable location of electrons cannot be precisely stated. The atom is such an important component of nature that many prominent scientists have theorized how it is made up.
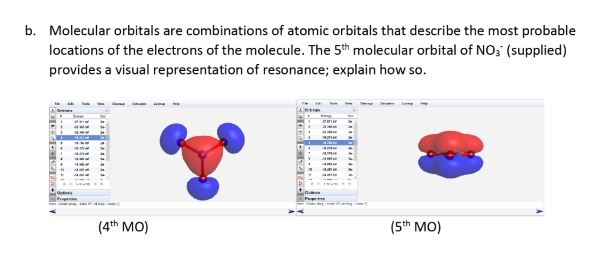
Solved B Molecular Orbitals Are Combinations Of Atomic Chegg Com
Used to describe the probable location of electrons in an atom.

. The discovery of subatomic particles -- protons neutrons and electrons -- did not settle the matter. Quantum numbers These four quantum numbers are used to describe the probable location of an electron in an atom. Region of space around the nucleus where an electron is likely to be found.
The first quantum number describes the electron shell or energy level of an atom. An η 3 ligand is trihaptic with three atoms sharing in the donation from the. Quantum numbers are important because they can be used to determine the electron configuration of an atom and the probable location of the atoms electronsThe magnetic quantum number m l describes the energy levels in a subshell and m s refers to the spin on the electron which can either be up or down.
Theoretical models do not correctly describe electron movements 2. Terms in this set 13 orbital. The location of an.
The three-dimensional region around the nucleus an orbital indicates the probable location of an electron. The describes the probable location of electrons in atoms. Are atoms of the same element that have different numbers of neutrons and.
The element hydrogen only has one orbital whereas radon has manyElectron cloud. The term orbital can be used to refer to the physical region where electrons can be found. This is called a probability distribution map a statistical representation of the probable locations of electrons as they exist in an atom.
Mathematically showed that waves can be used to describe the most probable location of electrons in atoms. Some common multihaptic ligands. 3-dimensional region around a nucleus that indicates the probable location of an electron.
Shortly after Bohr a man by the name of Heisenberg proposed an uncertainty principle which stated that it is impossible to know both the exact position and the exact velocity of a particle at the same time. The clouds of probability are the locations of electrons as determined by making repeated measurementseach measurement finds the electron in a definite location with a greater chance of finding the electron in some places rather than others. The Principal Quantum Number.
Arrenhasyd and 5 more users found this answer helpful. What term is used to describe splitting a large atomic nucleus into two smaller ones. A mathematical equation explained that a region in space around the nucleus is where electron can be found is called an.
They are designated with the names s p d and f. Other Apps - April 08 2022 Electron Probability Distribution For The Hydrogen Atom Wolfram Demonstrations Project Probability Electrons Hydrogen Atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom.
Formulated a mathematical equation that describe the behavior of the electron. The Locations of Protons Neutrons Electrons Within an Atomic Structure. Term Used to Describe Probabol Location of Electrons Get link.
What are some reasons scientists no longer use physical models to describe the motion of electrons. Quantum mechanics is a set of complex mathematics that is used to describe the most probable location of an electron. An orbital is a mathematical function that describes the wave-like behavior of electrons in an atom.
The three dimensional region around the nucleus of an atom that indicates the probability of the location of an electron is called an orbital. Previous models were physical 3The quantum mechanical 3 Schridunger devised a math equation that talks about the behavior of electrons. Different orbitals exist in atoms depending on the number of electrons the atom possesses.
In the early 1900s. An η 2 ligand is dihaptic with two atoms sharing in the donation from the pi system. In the drawings above the symbols η 2 or η 3 etc.
With the help of this function the probability of finding an electron in a given region is calculated. They make up the energy levels and are labeled s p d and f. A shorthand notation that shows the number and arrangement of electrons in an atoms orbitals orbital solved wave functions that describe a region of probable location of electrons.
Read eta-two or eta-three refer to the hapticity of the ligand.

Activity 2 Predicting The Probable Location Of An Electron By Hessmer Andre Sarajena

Electrons If All Atoms Are Composed Of The Same Fundamental Building Blocks How Is It That Different Atoms Have Vastly Different Chemically Behaviors Ppt Download

11 29 16 Today I Will Determine The Probable Location Of Each Electron In An Atom Warm Up Explain Bohr S Electron Energy Levels Ppt Download

Ch 13 Electrons In Atoms 13 1 Models Of The Atom Ppt Download
Using Schrodinger S Method Particle In A 1 D Box

Quantum Mechanical Model Overview History Expii

Electrons If All Atoms Are Composed Of The Same Fundamental Building Blocks How Is It That Different Atoms Have Vastly Different Chemical Behaviors Ppt Download
Using Schrodinger S Method Particle In A 1 D Box
Electron Configuration 4 Flashcards Quizlet

Q2 M1 Activity 2 Probable Location Of Electron Docx Name Grade And Section Date Teacher Activity 2 Predicting The Probable Location Of An Course Hero

Unit 3 Atomic Theory Quantum Mechanics Section A 6 A 7 Ppt Video Online Download
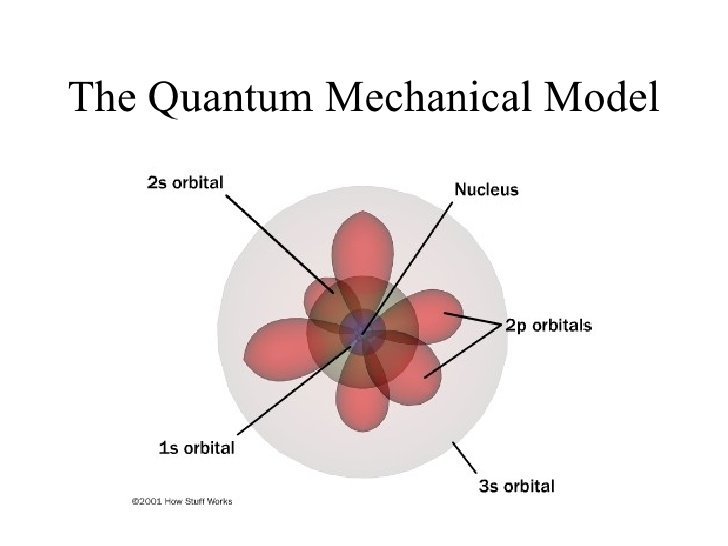
The Quantum Mechanical Model Of The Atom

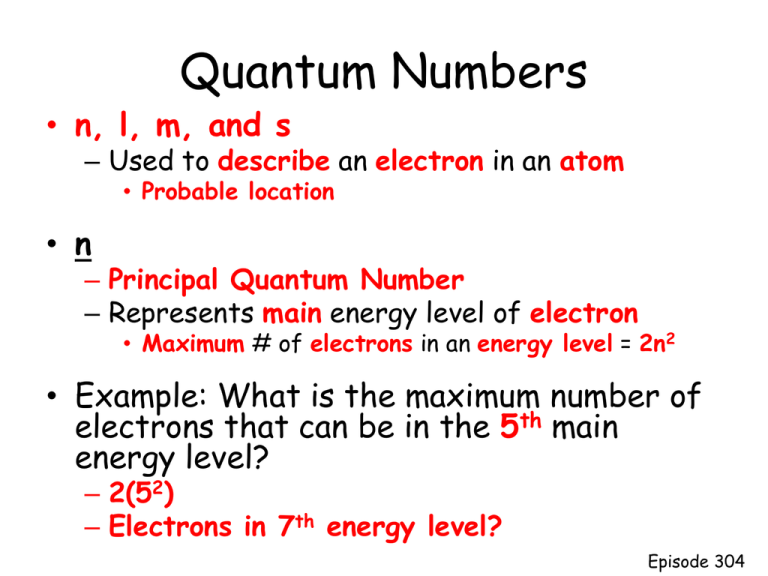
Quantum Numbers N L M And S Used To Describe An Electron In An Atom Probable Location N Principal Quantum Number Represents Main Energy Level Ppt Download
Quantum Mechanical Model Science Quizizz

Most Probable Location Of Electron In Hydrogen Atom Youtube

5 2 Quantum Theory Atom Ppt Download

Comments
Post a Comment